We want to empower our sales persons
If you can’t find your answer please reach out to us.
Commonly Asked Questions.
- Unlike Hillrom, we are interested in the comfort of the patients. Patients for the first time will experience comfort in a hospital mattress, something they don’t expect, but deserve.
- We are focused on innovating and using the latest foam technology. We have been focusing on developing more comfortable support surfaces, State of the art technology in the comfort that no-one else has.
- Our open-cell memory mattresses structure helps with airflow and lower temperature- resulting in more comfort. Unlike Gel mattresses, which assimilate the temperature of the person.
- Hillrom makes firmer mattresses, which are very uncomfortable for those of smaller stature.
And for this reason, we developed with the assistance of our chemical suppliers, an innovative High Resilience foam, a product with a density is 30 to 40% higher, and with low ILD (latex firmness) numbers - We ensure comfort and guarantee mattresses longevity for twice as long hospital current manufacturers.
- Although the quality of our mattresses is a higher cost to us, we have a better cost structure.
- Our mattress come standard with quality 2- and 4-way stretch materials, improving comfort.
- LIKE Hillrom, we are FDA approved and passed all fire barrier testing.
Searching the Internet for a foam deemed “medical grade” can be an intimidating and discouraging mission. This is especially true when the search results yield an overwhelming amount of similar buzz words to decipher and distinguish (i.e. medical foam, medical grade foam, foam used in medical products, etc.). The term “medical grade” foam means many things to many different people. Here at UFP Technologies, we follow a simple set of guidelines and definitions which allow us to determine the most functional and economical foam to use in our customers’ medical applications. Below are the most
common foam definitions we use to help select the correct material for your project:
- Biocompatibility
The foam has been tested, passed and certified to comply with the ISO 10993 protocol. - Fixed Formulation
The raw material formulation may be registered with the FDA. Should the material change, it is determined that the foam is not able to be categorized as a medical grade foam without additional testing. - FDA Master File
This is where foam formulations are registered with the FDA. It is important to remember that a foam with an FDA master file means that the formulation is registered with the FDA. It does not necessarily mean that the foam is “medical grade.” Additional biocompatibility testing will determine whether or not the foam is appropriate for use in medical applications. - 510K Submissions
We will work with the raw material supplier and customer to determine if the foam has previously been registered in a 510K application. - USP Classification
This is a raw material registration used primarily in the pharmaceutical industry.
UFP Technologies’ sales and engineering teams pair your project’s requirements with the above classifications to ensure that we are providing you with a superior, technical foam solution. We are then equipped to engineer and fabricate an innovative solution for you at one of our ISO 13485:2003 manufacturing sites. The ISO 13485:2003 certification is a method of systems and documentation to ensure that the manufacturer does not change the raw material or the methods of fabricating during the manufacturing process. Choosing to keep the production within an ISO 13485:2003 facility is becoming an industry standard. After all, the investment of time and economics has already been made to determine the appropriate medical grade foam.
Antimicrobial fabric, what is it? What’s it made of? Why is it effective?
What do you need to know about this specialty fabric and why is it great for use in so many products?
First, let’s define what antimicrobial means. The term ‘antimicrobial’ is defined as: “destroying or inhibiting the growth of microorganisms, and especially pathogenic microorganisms.”
Microorganisms “include bacteria, viruses, protozoans, and fungi, like mold and mildew.” Antimicrobial products are common in medical facilities and are used in textiles. At first, it might seem strange that antimicrobials are found in fabrics, but the truth is, without this layer of protection, many fabric products would succumb to contamination and have to be discarded. Here are is a quick list of where you might find an antimicrobial fabrics.
- Medical bedding
- Medical curtains
- Uniforms – military, hospital, and more
When it comes to specialty fabrics and the use of antimicrobial features, it’s important to understand that including a pathogen fighting layer of defense prolongs the life of the textile and protects the end user.
Basically, an antimicrobial is applied to a fabric to help fight off pathogens that could potentially infect a patient. This means that the antimicrobial is constantly working against microorganisms and not only protecting human users, but also prolonging the life of the fabric. For medical facilities, investing in antimicrobial textiles is one way to ensure the longevity of the fabric and also help keep replacement costs down.
Antimicrobial fabrics can be made of a variety of textiles, including but not limited to polyester, polyester-vinyl composites, vinyl, and even acrylics. The effectiveness of an antimicrobial fabric lies in its ability to fend off microorganisms, and its ability to help prolong the life of a textile. Think about hospital blankets, bedding, and even mattresses. These are constantly imbued with sweat, oils, and other contaminants, and can easily become breeding grounds for bacteria, mold and mildew. But, through the use of an antimicrobial, coupled with the application of other features – like flame, stain and odor resistance, and waterproofing – the fabric can withstand regular wear and tear and last far longer than expected.
Similar to antibacterial soaps and disinfectants, antimicrobial fabrics help reduce the spread of disease. The primary function of antimicrobials is to help prevent bacteria and other microorganisms from attaching to the fabric surface. Keep in mind, microorganisms can live in a fabric and grow. That’s why antimicrobials are so effective — they prevent the growth and spread of microorganisms within the fabric. This is especially useful in the healthcare industry where exposure to bacteria and other pathogens is possible on a daily, hourly, basis. From beds to pillows, to hospital gowns, and even the scrubs a nurse or doctor wears, fabrics in the healthcare industry must be designed to prevent the spread of disease. That’s why the use of antimicrobial fabrics is so common and so helpful in the places like hospitals.
Visco Elastic Foam
Visco elastic foam is a polymer created during a chemical reaction between polyol and diisocyanate (two chemicals created from organic compounds). The ratio of polyol to diisocyanate is 2:1 to form polyurethane, though there may be other compounds that are added to create just the right amount of elasticity and density for each piece of foam. When the chemical reaction occurs in the presence of water with catalysts like tin and amines, a new viscous liquid in the consitency of a ice cream is formed. This hot mixture is then placed in a mold, dried, cooled, and cut for the use in pillows and mattresses. For most foam manufacturers, this end-to-end process of catalyzing the foam to clipping it for a mattress is about 8 hours.
How it is Used in Bedding
Visco elastic foam has wide spread use in pillows, toppers, and mattresses. Though visco elastic foam is an industry buzzword, it is usually found only in the top layer of visco elastic foam mattresses. Other layers of ‘visco elastic foam’ mattresses are usually a blend of polyurethane foams with less elastic attributes.
Visco elastic foam is another term for memory foam. It is a blend of polyurethane foam that was developed for airplane cushions by a NASA contract in the 1970s.
The difference between visco elastic foam and other types of polyurethane foam is its slow response and low resistance. Its soft surface allows for contouring and its slow response lowers bounce.
Visco elastic foam, like all polyurethane foam, is made from a blend of substances and has a tendency to off-gas. Those with lung sensitivities should do additional research about the quality of foam to ensure that it won’t aggravate them.
Pros: Contouring comfort, competitive price-points, and widespread options.
Cons: Sagging and off-gassing problems with lower quality foams.
Material Scores
Overall Score: 9.3/10
Comfort: 9.4/10
Softness: 9.5/10
Heat: 8/10
Hypoallergenic: 8.1/10
Odor: 8.4/10
Eco-Conscious: 8.5/10
General Support: 8.7/10
Price Value: 9.4/10
If you were to tell a stranger that foam has density, weight, and firmness characteristics, he or she would likely understand, given how common the terms are. However, one of the most confusing things about foam is the relationship between these characteristics. On the surface, it would seem the density or weight of a material would allow you to draw a correlation about its firmness, and vice versa. In general, this is often true, but when applied to foam products, density and firmness are independent values for determining a foam’s qualities.
It would be accurate to say density is a foam characteristic that is “over-applied,” rather than one that is misunderstood. The density of foam means the same thing as any other application of the term; the quantity or mass of a material per a measurable size or volume. This pertains to all varieties of foam, including expanded polystyrene (EPS), polyethylene, polyurethane foam, and others. How density is measured varies across materials though, and in the case of foam, density is found by weighing a 12″ x 12″ x 12″ block of the material. If a product has a 3LB density, that means its 12″ x 12″ x 12″ block weighed 3LB. And while it’s vital to understand that density does not pertain to the firmness of a foam product, it does correlate to the quality and longevity of a product.
Many conventional foams have a density between 1LB and 3LB. However, the densest materials can be as much as 10 or 15LB. High-density foam, Like The Foam Factory’s 2.8LB density HD36-HQ foam, is optimal for uses that receive heavy or daily use like couch cushions, bedding, or automobile seating. Lower density foam is excellent for occasional-use products like shipping foam, crafts, or guest room mattress toppers.
Density is also sometimes referred to as weight, which is a more literal translation of the characteristic given the testing process. But because of this, it’s always important to specify whether you want to know a product’s overall weight, or its density weight. Consider a 6-inch thick, conventional foam queen mattress with a 2.8LB density. The material weight is correctly stated as 2.8LB, since that’s its density. However, the overall weight of the mattress would be about 46LB. That’s about 43LB worth of reasons to make sure you clarify which value you need to know, since both can be technically correct.
Firmness meanwhile, interprets the feel of foam and how it yields to weight and pressure. Its measurement is called Indentation Load Deflection (ILD) (also known as Indentation Force Deflection/IFD), found by mechanical performance testing. A foam sample measuring 15″ by 15″ by 4″ is used and the force in pounds that it takes a 50 square inch circular indenter to compress the material 1″ (25 percent of its thickness) is recorded. If the sample requires 36LB of pressure to indent it 1″, its ILD is 36. It is also important that the test material meets the standardized dimensions, as different thicknesses of the same material can support weight differently. A hard foam material will require greater force to reach 25 percent compression, and a softer material will require less. Most common materials have ILD values from 8 to 70, with some materials as high as 120 to 150. A low ILD example would be The Foam Factory’s 12ILD Super Soft Foam, while their Rebond Foam is very firm at 70ILD.
Firmness testing is done to help illustrate how a material will bear weight in end-use applications. It is important to interpret firmness values as an explanation of a material’s physical feel rather than its quality, which is reflected by its density. Because of the numerous structural and chemical makeups of foam, some foam sheets with higher densities can even have a lower ILD than foams with lower densities. For this reason, the two values should be looked at independently and used to help find a product that matches your preferences.
Understanding what these characteristics do and do not tell you about a foam material is very important for selecting the perfect product for an application. By understanding the values of these measurements, you can have a better idea of what to expect from a product and make a more educated purchase.
10. Termination
10.1. Termination on Notice. Either party may terminate this agreement for any reason upon 90 days’ Notice to the other party.
10.2. Termination on Breach. If either party commits any material breach or material default in the performance of any obligation under this agreement, and the breach or default continues for a period of 20 Business Days after the other party delivers Notice to it reasonably detailing the breach or default, then the other party may terminate this agreement, with immediate effect, by giving Notice to the first party.
10.3. Termination on Insolvency. This agreement will terminate immediately upon either party’s insolvency, bankruptcy, receivership, dissolution, or liquidation.
10.4. Probationary Period. Representatives shall be subject to a 90-day probationary period. If at any time during this period AHS, in its sole discretion, determines that the Representative is unsuitable for the position for which he or she has been hired, AHS may immediately terminate the Representative’s employment, paying the Representative through the date and time of terminating. Any such termination shall not affect the Representative’s continuing obligating as set forth in this Agreement, including, but not limited to, Paragraph 9 above. AHS may also at its discretion extend the probationary period for up to an additional 90-days. At the end of the probationary period or as soon thereafter as a reasonably practicable, AHS will discuss the Representative’s performance with Representative and inform him or her that he or she has completed the probationary period. Representatives within their probationary period shall not be eligible for any benefits and shall receive no benefits or pay in lieu of benefits if their employment is terminated prior to the term or extended term of their probationary period. The preceding clause supersedes all other terms of this contract having to do with benefits.
SPECIALTY FOAMS
There are many foam materials that are manufactured to solve very specific problems. Ranging from conductive foams used for packaging electronic devices to control static, hydrophilic foams to absorb ink in a printer, and breathable foams to provide comfort to a patient after surgery, all of these problems are solved by UFP’s experience with specialty foams.
Our access to unique materials allows us to provide you with the perfect material to meet your specific requirements. Below is a sampling of some of the specialty foam materials that we have access to and expertise in fabricating into components, packaging and products.
Anti-static
A grade of foam that prevents or inhibits the build up of static electricity, primarily used for the protection and packaging of sensitive electronic components and products. This offers a lower range of electrical resistance than static dissipative and conductive foams.
Conductive
A grade of foam that offers the highest level of electrical resistance, and provides excellent EMI shielding. Conductivity refers to the material’s ability to drain away charges instantly.
Static Dissipative
A grade of foam in the middle range of electrical resistance greater than anti-static and lower than conductive.
Breathable
Open cell foams that allow air to pass through at controlled and measurable rates.
Hydrophilic
A “water loving” grade of foam for applications that require the ability to absorb or retain moisture.
Neoprene
A synthetic rubber product from DuPont of high molecular weight. Its ability to stretch, and resistance to chemicals and oxidation, make it ideal for hoses, adhesives, and many other applications. UFP solutions will often combine Neoprene with a wide array of fabrics.
Facings
UFP Technologies has the capability to apply facings to a host of foam materials that we fabricate. A common foam facing would be reinforced aluminum foil that can be applied to most foams to provide a protective, wipe-able, or heat reflective surface.
Return Policy
No matter the reason, Austin Healthcare Solutions is here to help with our 30-day return policy for easy returns and free exchanges. For the large majority of our products, we offer a 30 day return policy. You may accept a refund of the purchase price less the restocking fee (20%). Please refer to the individual products warranty specifications for the return policy of your particular product.
To return a product a Customer Service Representative must first authorize the transaction and issue an RMA number (Return Merchandise Authorization). The following information should be available and provided by the purchaser at the time of consultation:
- Reason for Return
- Product Name/Model
- Product Quantity
- Customer Name and Invoice Number
Eligibility/Guidelines/Exclusions
- Items must be returned in original packaging and in brand new condition within 30 days of receiving the item.
- The customer is responsible for shipping expenses and restocking fee on all eligible items.
- Unused goods in original packaging receive an 80% credit with the customer being responsible for the cost of shipping.
- We will not issue an RMA on items that are non-refundable, such as custom or special order items, or in the case that an item is danged due to improper handling/packaging by the customer.
- We do not accept returns for replacement parts for any reason other than an error on our part.
- No credit will be given for items returned without an RMA number. If the return isn’t honored the item may be returned at the customers expense.
- Product modifications made by the customer or contracted parties (made by the customer) surrender their ability to return the product due to the compromise of the products condition.
RETICULATED POLYURETHANE FOAM
Reticulated polyurethane foam is a versatile, open-cell material that is light weight, low odor and highly resistant to mildew. It’s typically used to make products that are involved in filtration, sound absorption, fluid management, wiping and padding. This family of materials features high tensile, elongation and tear characteristics.
UFP Technologies has designed and fabricated a host of solutions using reticulated foams. This includes liquid delivery components for medical devices, sound absorption components in automobiles, cosmetic applicators, air conditioner filters, and sponges. Reticulated polyurethane has a high resistance to chemicals, which makes it an ideal material for filtration applications such as in lawn mower engines or wipes used in clean rooms.
The porosity of reticulated foams is vital when designing a custom component or product. UFP Technologies’ technical expertise will help guide you when selecting the proper material for your component.
Foam technology involves the manipulation of thousands of plastic bubbles (called cells) of precisely controlled sizes. Reticulation is a post process in foam manufacturing that removes the window membranes of the cell. The cells that make up the foam can have a number of variations, which can also be precisely controlled. Different foams have varying cell structures and characteristics, but foams from the same material family can also be made with vastly different density and firmness specifications that will greatly affect their performance.
Applications
Reticulated foam is available in two primary types, polyether and polyester and a range of densities and colors. A fine porosity, 100 PPI (pores per inch) reticulated foam is used in a wide variety of applications such as:
- Sound absorber in anechoic chambers
- Microphones
- Windscreens
- Filters
- Face masks
- Wiping pads and applicators
Reticulated foam can act as a filter. Filter foam is a reticulated polyurethane foam, specially adapted to air and liquid filtration, in a range of controlled cells from 10 pores per inch to 100 PPI. Filter foam, with a medium porosity 45 PPI works as a depth loading filter, opposed to a surface loading filter, trapping dust particles within its cell structure. Because the reticulation process leaves behind the skeletal structure of the foam it is 97% void volume giving it a high degree of surface area for impingement of dust particles. With its homogeneous and uniform cell structure, a reticulated foam filter can be engineered for pressure drop and filtering efficiency by changing its pore size. A coarse porosity, 10 PPI, reticulated foam is effective as a sound attenuator, scrubber pad, washable filtration media for air conditioners, furnaces, small engines and automobile air cleaners, plus many other applications.
When reticulated foam is compressed, the material takes on a new set of properties ideal for other applications requiring high void volumes, uniform porosity, non-directional characteristics, exceptional breathability and uniform texture. Reticulated foam is often compressed and used for creating ink rollers, blood filters, and other products requiring wicking properties.
Polyester Foam
Reticulated polyester urethane foams have a three-dimensional skeletal strand structure that minimizes the possibility of open channels and provides excellent filtration properties. Polyester foam is a flexible, open-cell type of polyurethane foam that is porous and has a uniform cell structure. It has an evenly spaced cell structure with a high proportion of closed cells or “windows” that reflect light. The large amount of windows makes polyester foam ideal for sealing as it naturally prohibits the flow of air. The uniform cell structure inherent in polyester foam also makes it ideal for the reticulation process. Polyester foam can be easily compressed, forming sheets with a fixed, desirable thickness. Additives introduced during manufacturing can transform the foam properties making it flame retardant, anti-microbial, anti-static, conductive and/or electrostatic dissipative (ESD). Felted polyester filter foam has a highly restrictive cell structure and is largely used for air and liquid filtration.
Polyether Foam
Reticulated polyether urethane foams were developed for increased hydrostatic stability. Polyether foam is a flexible, compressed, open-cell type of polyurethane foam that is smooth in texture. Polyether foam is manufactured by mixing polyether polyols with catalysts and a blowing agent, forming a free-rising, foamy froth that solidifies within minutes resulting in a slab stock bun of polyether foam ready for fabrication. The protective and cushioning properties of polyester foam make it an ideal material for packaging applications. It has a high viscosity rate, superior solvency qualities and is resistant to abrasion and cutting. It is used widely in the medical, apparel and sports industries.
Polyethylene Foam – Foam Fabrication
Polyurethane Foam
Polyurethane foam is a blanket term that includes any foam that is synthesized using polyol and diisocyanate. These include but are not limited to: memory foam, high resilience foam, and high density foam. Since polyurethane foam can contain additives, a manufacturer that comes up with a new formula can name the new foam whatever they like. Many memory foam mattresses are a combination of polyurethane foams, layered from dense on the bottom to less dense on the surface layers.
Polyurethane is a synthetic blend of organic compounds and has a tendency to off-gas. If you are susceptible to lung issues, please do additional research on the quality of foams before purchasing.
Polyethylene foam is a durable, lightweight, resilient, closed-cell material. It is often used for packaging fragile goods due to its excellent vibration dampening and insulation properties. It also offers high resistance to chemicals and moisture. It is easy to process and fabricate. It has high load bearing characteristics that help manufacturers reduce packaging costs as they can use thinner and smaller amounts of foam yet still protect their products.
UFP Technologies can recommend the proper polyethylene foam material for your unique packaging, component, or product. Our engineering team will work with you to design your solution and manufacture the final parts. Polyethylene Foam Material Characteristics:
- Closed-Cell
- Very lightweight
- Non-abrasive
- Easy to fabricate
- Non-Dusting
- Superb strength and tear resistance
- Excellent shock absorption & vibration dampening properties
- Flexibility
- Impervious to mildew, mold, rot, and bacteria
- Resistant to water, chemicals, solvents & grease
- CFC free
- Odorless
- Excellent buoyancy
- Very cost-effective
- Excellent thermal insulation properties
Pros: Many different types of foams exist under the polyurethane umbrella — some are excellent for bedding and offer different firmness varieties!
Cons: Off-gassing and durability problems with lower quality foams.
ARTICLE WORTH READING:
1.4. No Commissions in Certain Circumstances. The Company will not be required to pay the Representative a commission in any of the following circumstances:
a) if prohibited under applicable Law,
b) if the Representative did not directly facilitate the sale of the Products to a customer,
c) on any sale to any customer that is directly or indirectly owned by or under common ownership with the Representative,
d) on any sales outside of the Territory, or
e) on any sale of Products to a customer occurring after the expiration or termination of this agreement, unless the sale is the direct result of the Representative’s sales efforts before the termination or expiration.
1.5. Expenses. The Representative is solely responsible for any expenses it incurs in performing its services under this agreement. Unless previously approved by AHS.
1.6. Definition of “Net Amount.” In this agreement, “Net Amount” means the sales price of the sold product as listed on the applicable invoice, less charges may include, but not limited to, handling, freight, sales, use, value added, or similar taxes, import or export taxes or levies taxes, C.O.D. charges, insurance, customs duties, trade discounts, and any other fees or charges of any Governmental Authority.
MELAMINE FOAM
UFP Technologies is one of the few companies in the world with access to BASF’s product line of melamine foam. It is most commonly used as a cleaning scrubber for popular eraser products found in retail stores.
Material Properties:
- Light-weight
- Flame resistant
- Retains properties from -200⁰C to 240⁰C
- Sound absorption
- Abrasiveness
- Thermal insulation
- Chemical resistance
Its diverse properties allow it to be used in numerous applications and industries, such as:
- Consumer:
- Cleaning sponges and erasers
- Construction & Industrial:
- Acoustics: ceiling panels, wall panels, suspended baffles
- Insulation: pipe jackets, thermal tanks, solar collectors, HVAC systems
- Medical:
- Insulation and sound absorption for medical equipment
- Semiconductor:
- Semiconductor wafer equipment cleaning
- Transportation:
- Aviation: cabin insulation and ductwork
- Cars and buses: engine covers, firewalls, transmission tunnels, hood liners
- Rail: ceiling panels
Our design and engineering expertise coupled with our diverse fabrication capabilities allow us to solve your complex product challenges.
WARNING: All polyurethane foams including “FR” types will burn. Do not expose to any flame source.
Once ignited, they can produce rapid flame spread, intense heat, dense smoke and toxic gases causing death. Warnings should be given to your employees and customers. Test data does not necessarily reflect a foam’s performance under actual fire conditions.
Properties that Affect Foam Performance
There are a number of physical properties of flexible polyurethane foam that can be used when selecting foam cushioning for different applications. Following is a brief description of the physical properties of foam, and the importance of each. Physical properties of foam are measured under closely controlled conditions of humidity and temperature. Care must be taken to reproduce those conditions when testing samples of foam for physical properties.
Density
Measured and expressed in pounds per cubic foot (pcf), density is one of the most important of all foam properties. Density is a function of the chemistry used to produce the foam and additives included with the foam chemistry.
Density affects foam durability and support. Typically, the higher the density, the better the foam will retain its original properties and provide the support and comfort it was originally designed to produce.
IFD (Compression)
Indentation Force Deflection (IFD) is a measurement of foam firmness. Firmness is independent of foam density, although it is often thought that higher density foams are firmer. It is possible to have high density foams that are soft-or low density foams that are firm, depending on the IFD specification. IFD specification relates to comfort. It is a measurement of the surface feel of the foam.
IFD is measured by indenting (compressing) a foam sample 25 percent of its original height. The amount of force (in pounds) required to indent the foam is its 25 percent IFD measurement.The more force required, the firmer the foam. Flexible foam IFD measurements range from about 10 pounds (supersoft) to about 80 pounds (very firm).
Resilience
Resilience is an indicator of the surface elasticity or “springiness” of foam. Resilience can relate to comfort. Resilience is typically measured by dropping a steel ball onto the foam cushion and measuring how high the ball rebounds. Higher resilience in a foam often means that sofa seat cushions, for example, have a better “hand” or surface feel.
Closed-Cell Cross-Linked Polyethylene Foam
Cross-linked polyethylene foam (also known as XLPE) is a closed-cell foam characterized by a compact feel and resistance to water. It has many of the same properties as polyethylene foam but also has the ability to protect Class “A” surfaces and is consequently used extensively in the packaging of medical products and equipment.
Compression molding and thermoforming are common fabricating methods used when creating components, packaging and products from closed-cell cross-linked polyethylene foams. These fabrication methods provide a unique finish that is aesthetically pleasing while still providing the necessary cushioning and support required.
UFP Technologies designs and fabricates custom packaging, components, and products utilizing closed-cell cross-linked polyethylene foams.
Closed-cell cross-linked polyethylene foams are commonly used in the following applications:
- Medical device packaging
- Military grade tool control
- Orthopedic soft goods
- Athletic padding/helmet lining
- Protective case inserts
Closed and Open Cell Foams For Medical Applications
As one of the leading medical foam fabricators in North America, UFP Technologies has access to an array of closed-cell and open-cell foams suitable for a variety of medical applications.
UFP Technologies has multiple ISO 13485 certified facilities throughout the country housing clean rooms and controlled environments, equipped with advanced foam fabrication capabilities, including compression molding, CNC routing, die-cutting and more.
Our knowledge of materials, design expertise and manufacturing capabilities allow us to work with customers to design and develop custom innovative solutions for the medical industry.
Closed-Cell Medical Foams
Our closed-cell foams such as polyethylene foams and cross-linked polyethylene foams are commonly used for medical device packaging and component solutions.
UFP Technologies utilizes cross-linked polyethylene foams that are impregnated with nitrogen providing a non-toxic, hypoallergenic, latex-free solution that can come in direct contact with skin and be used in operating rooms. We use these materials in solutions ranging from:
- Medical device packaging
- Orthopedic soft goods
- Medical components
Open-Cell Medical Foams
UFP Technologies offers a range of medical grade open-cell foams such as polyurethane and reticulated polyurethane foams that are frequently used for various medical applications and packaging solutions.
Reticulated polyurethane foams are used extensively in the medical industry as they are easy to clean, impervious to microbial organisms, and can be made with fungicidal and bactericidal additives for added safety. Reticulated polyurethane foams can even be injected with substances to achieve other unique properties. Common uses include:
- Molded covers for prosthetic devices
- Specialty sponges
- EKG pads, filters and sterilization bags
1.2. Calculation of Commissions. Compensation shall be commission only. Commissions shall be payable to the Representative for all net billings, for all orders of those products solicited directly by the Representative. AHS reserves the right to determine the Representative’s ownership of each order, at its sole discretion. Such orders must be delivered and billed to the customer with the territory described herein, when said products are sold at AHS’s current list price, or such other promotional prices as AHS shall specify in writing from time to time. AHS’s current list price is defined as the selling price, which is currently in AHS’s “Inventory Item File” of it computer system upon the date of receipt of the customer’s bonafide purchase order. “AHS’s Promotional Prices” is defined as the selling price, which is currently advertised in AHS’s catalogs and/or other promotional literature, and which at AHS’s sole discretion is currently in effect upon the date of receipt of the customer’s bonafide purchase order.
The Commission rate for sales in each month during which the Representative actively works shall be paid according to the following schedule.
- Sales equal to, or less than $15,000 per month =15% commission
- Sales from $15,001 to $20,000 per month =18% commission
- Sales exceeding $20,000 per month = 20% commission
Representatives shall be entitled to payment of commission at the above rates, provided that each sale on which commission is to be paid is in strict compliance with AHS Authorized Prices. If a sale does not meet AHS’s terms of sale or is made for a different price than AHS’s Authorized Price for the particular product or products, AHS may, at its sole discretion, either reject the order or accept the order while deduction any added cost or pricing deficiency from any payment due to the Representative.
1.3. Offsets and Charge-Backs. In calculating the Representative’s commission, the Company may offset any credits, cancellations, refunds, allowances, and returns to or by customers of revenues on which Representative has already been paid commissions under this agreement, but in no event will the offset for any customer exceed the sales price of that customer’s returned, cancelled, or otherwise credited Products.
1.4. No Commissions in Certain Circumstances. The Company will not be required to pay the Representative a commission in any of the following circumstances:
- if prohibited under applicable Law,
- if the Representative did not directly facilitate the sale of the Products to a customer,
- on any sale to any customer that is directly or indirectly owned by or under common ownership with the Representative,
- on any sales outside of the Territory, or
- on any sale of Products to a customer occurring after the expiration or termination of this agreement, unless the sale is the direct result of the Representative’s sales efforts before the termination or expiration.
1.5. Expenses. The Representative is solely responsible for any expenses it incurs in performing its services under this agreement. Unless previously approved by AHS.
1.6. Definition of “Net Amount.” In this agreement, “Net Amount” means the sales price of the sold product as listed on the applicable invoice, less charges may include, but not limited to, handling, freight, sales, use, value added, or similar taxes, import or export taxes or levies taxes, C.O.D. charges, insurance, customs duties, trade discounts, and any other fees or charges of any Governmental Authority.
Gowns are examples of personal protective equipment used in health care settings. They are used to protect the wearer from the spread of infection or illness if the wearer comes in contact with potentially infectious liquid and solid material. They may also be used to help prevent the gown wearer from contaminating vulnerable patients, such as those with weakened immune systems. Gowns are one part of an infection-control strategy.
A few of the many terms that have been used to refer to gowns intended for use in health care settings, include surgical gowns, isolation gowns, surgical isolation gowns, nonsurgical gowns, procedural gowns, and operating room gowns.
In 2004, the FDA recognized the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70:2003, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.” New terminology in the standard describes the barrier protection levels of gowns and other protective apparel intended for use in health care facilities and specifies test methods and performance results necessary to verify and validate the newly defined levels of protection:
- Level 1: Minimal risk, to be use used, for example, during basic care, standard isolation, cover gown for visitors, or in a standard medical unit
- Level 2: Low risk, to be use used, for example, during blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab
- Level 3: Moderate risk, to be use used, for example, during arterial blood draw, inserting an Intravenous (IV) line, in the Emergency Room, or for trauma cases
- Level 4: High risk, to be use used, for example, during long, fluid intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne)
Surgical Gowns
A surgical gown is regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter. Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards. As referenced in Figure 1: the critical zones include the chest from scapula to knees and sleeves from cuff to above the elbow. Surgical gowns can be used for any risk level (Levels 1-4). All surgical gowns must be labeled as a surgical gown.
Surgical Isolation Gowns
Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones than traditional surgical gowns. Surgical isolation gowns, like surgical gowns, are regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. As referenced in Figure 2, all areas of the surgical isolation gownexcept bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown. Additionally, the fabric of the surgical isolation gown should cover as much of the body as is appropriate for the intended use.
Product names may include but are not limited to isolation gown, procedure gown, or protective gown. Since names are not standardized, product labeling that describes its intended use for isolation precautions or liquid barrier protection in moderate or high risk situations fall into this category.
Non-Surgical Gowns
Non-surgical gowns are Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations. Non-surgical gowns are not worn during surgical procedures, invasive procedures, or when there is a medium to high risk of contamination.
Like surgical isolation gowns, non-surgical gowns should also cover as much of the body as is appropriate to the task. As referenced in Figure 2, all areas of the non-surgical gownexcept bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown.
Product names may include but are not limited to isolation gown, procedure gown, or cover gown. Since names are not standardized, when choosing these gowns look for product labeling that describes an intended use for protection in minimal or low risk situations.
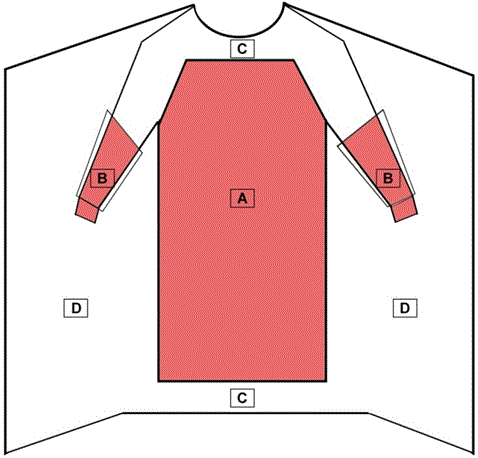
Figure 1 – Critical Zones for Surgical Gowns
- The entire front of the gown (areas A, B, and C) is required to have a barrier performance of at least level 1.
- The critical zone compromises at least areas A and B.
- The back of the surgical gown (area D) may be nonprotective.
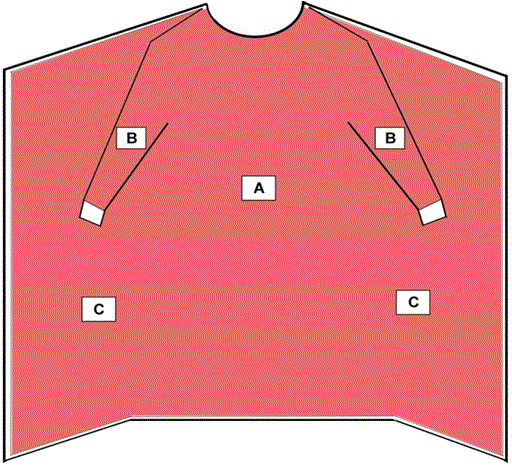
Figure 2 – Critical Zones for Surgical Isolation Gowns and Non-Surgical Gowns
- The entire gown (areas A, B, and C), including seams but excluding cuff, hems, and bindings, is required to have a barrier performance of at least Level 1.
- Surgical isolation gowns are used when there is a medium to high risk of contamination and need for larger critical zones than traditional surgical gowns.
Standards for Gowns
Labeling that shows a product has been tested to and meets appropriate performance standards is one way for users and procurers to determine when to use a particular gown.
The performance of gowns is primarily tested using two consensus standards:
American Society for Testing and Materials (ASTM) F2407 is an umbrella document which describes testing for surgical gowns: tear resistance, seam strength, lint generation, evaporative resistance, and water vapor transmission.
Below is a summary of ASTM F2407 standard recognized by the FDA.
- Tensile Strength, ASTM D5034, ASTM D1682
- Tear resistance : ASTM D5587(woven), ASTM D5587 (nonwoven), ASTM D1424
- Seam Strength: ASTM D751 (stretch woven or knit)
- Lint Generation (ISO 9073 Part 10)
- Water vapor transmission (breathability) ASTM F1868 Part B, ASTM D6701 (nonwoven), ASTM D737-75
American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI): ANSI/AAMI PB70:2003 describes liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.
Below is a table summarizing the ANSI/AAMI PB70 standard recognized by the FDA.
Type of PPE: Gowns
Feature Tested: Liquid Barrier Performance
Standard Designation: AAMI PB70:2012
Classifies a gown’s ability to act as a barrier to penetration by liquids or liquid-borne pathogens based on four levels.
The critical protective zones for surgical and non-surgical gowns are defined differently by the standard.
While the critical zones designate different protective areas for the different gowns, the levels of protection are the same for both surgical and non-surgical gowns
LEVEL1: Basic care, standard hospital medical unit
Liquid barrier performance is not related to the strength of the material.
- Used for MINIMAL risk situations
- Provides a slight barrier to small amounts of fluid penetration
- Single test of water impacting the surface of the gown material is conducted to assess barrier protection performance.
LEVEL 2: Blood draw from a vein, Suturing, Intensive care unit, Pathology lab
- Used in LOW risk situations
- Provides a barrier to larger amounts of fluid penetration through splatter and some fluid exposure through soaking
- Two tests are conducted to assess barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
LEVEL 3: Arterial blood draw, Inserting an IV, Emergency Room, Trauma
- Used in MODERATE risk situations
- Provides a barrier to larger amounts of fluid penetration through splatter and more fluid exposure through soaking than Level 2
- Two tests are conducted to test barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
LEVEL 4: Pathogen resistance, Infectious diseases (non-airborne), Large amounts of fluid exposure over long periods
- Used in HIGH risk situations
- Prevents all fluid penetration for up to 1 hour
- May prevent VIRUS penetration for up to 1 hour
- In addition to the other tests conducted under levels 1-3, barrier level performance is tested with a simulated blood containing a virus. If no virus is found at the end of the test, the gown passes.
Conformance with recognized consensus standards is voluntary for a medical device manufacturer. A manufacturer may choose to conform to applicable recognized standards or may choose to address relevant issues in another manner.